Renolure DERM Biphasic Hyaluronic Acid Dermal Filler
Name: Renolure DERM
Attribute: Crosslinked Hyaluronic Acid Injectable Filler
Needle Size: 27Gx1pcs +26Gx1pcs
HA Concentration: 24mg / ml
Particle size: 0.15-0.28mm
- Overview
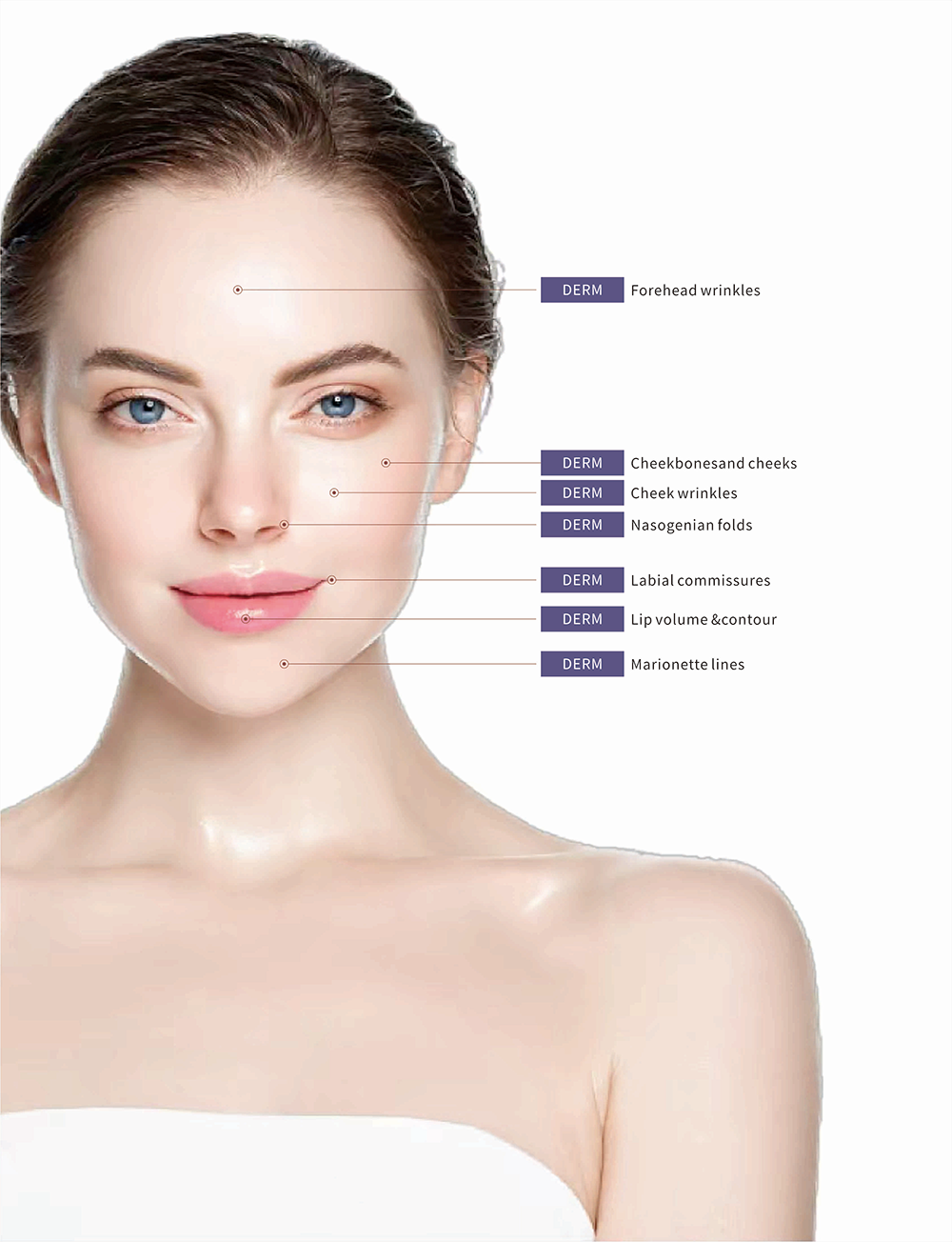
Renolure DERM dermal fillers is used for correction of fine lines and wrinkles or facial defects
Renolure cross-linked sodium hyaluronate gel for plastic surgery can enlarge the volume of subcutaneous tissue. It absorbs water from surrounding tissue to expand volume, then make the relaxation skin plump again.
Secondly, it can be used to treat and repair aging skin folds, depression or scar and can also augment facial features sub as the cheeck and lips. It can also be used for therapeutic applications: correct the defects that result from medical disorders, trauma, or surgery.And it can also fill the HIV-associated lipoatrophy, scaring, and surgical or traumatic skull defects, correct facial asymmetry (e.g. lip asymmetry after surgical correction of cleft lip and eyelids malposition).
| Name | Renolure DERM |
| Attribute | Crosslinked Hyaluronic Acid Injectable Filler |
| Needle Size | 27Gx1pcs +26Gx1pcs |
| HA Concentration | 24mg / ml |
| Particle size | 0.15-0.28mm |
| Insertion Depth | surperfacial and middle dermis |
| Effect Duration | 6-10 months |
| Storage Condition | Store at 2-30C.Do not freeze. Protect from light source. |
| Indications for use | It's used for lips defect and augmentation. |
Contraindications
• Patients with a history of hypertrophic or keloid scarring or with a current streptococcal infection, active skin disease, inflammation or other infections.
• Patients with a history of autoimmune disease or who are receiving immune therapy.
• Patients who are known to be hypersensitive to hyaluronic acid.
• This product has not been tested on pregnant or breastfeeding women, or on children. There is therefore no evidence for it’s safety in these groups.
• The existing clinical data has confirmed the safety and effectiveness of this product for use in adults.There are no clinical data available for using the product in people under the age of 18. Therefore, the product is not recommended for such group.
• Anti-coagulated patients or patients receiving platelet aggregation inhibitors (e.g. ASS) should consult their doctors.
• The product should be kept out of reach for children.
• Do not use in patients with bleeding disorders.
• Do not use in patients who are taking thrombolytics or anticoagulants, or have taken inhibitors of platelet aggregation in the preceding 2 weeks.
• Do not use in patients with hyperplastic scar and streptococcal infection.
• Do not use the product together with a laser treatment, chemical peeling, intensive pulse light or dermabrasion treatment.
• The product should not be used in or near anatomic sites where there is active skin disease, inflammation, infection or related conditions.
• Do not implant the areas where injection of other products took place.
Anticipated Adverse Events
Common and short-term, self-limiting reactions after injection include erythema, swelling, pain, itching and discoloration.
This product equilibrates to the normal pressure of the tissues. As the pressure of the tissues is sometimes increased (in the case of oedema) or decreased (in the case of dehydration), a small change in appearance may occur in these circumstances. In more serious cases, a short course of oral steroids can be helpful. Patients presenting with this kind of reaction must not be treated again with this product.
Complication
The estimated incidence of these complication is 0.05%( 1 in2000 patients),which classifies them as to rare events. Noserious adverse events have been reported.