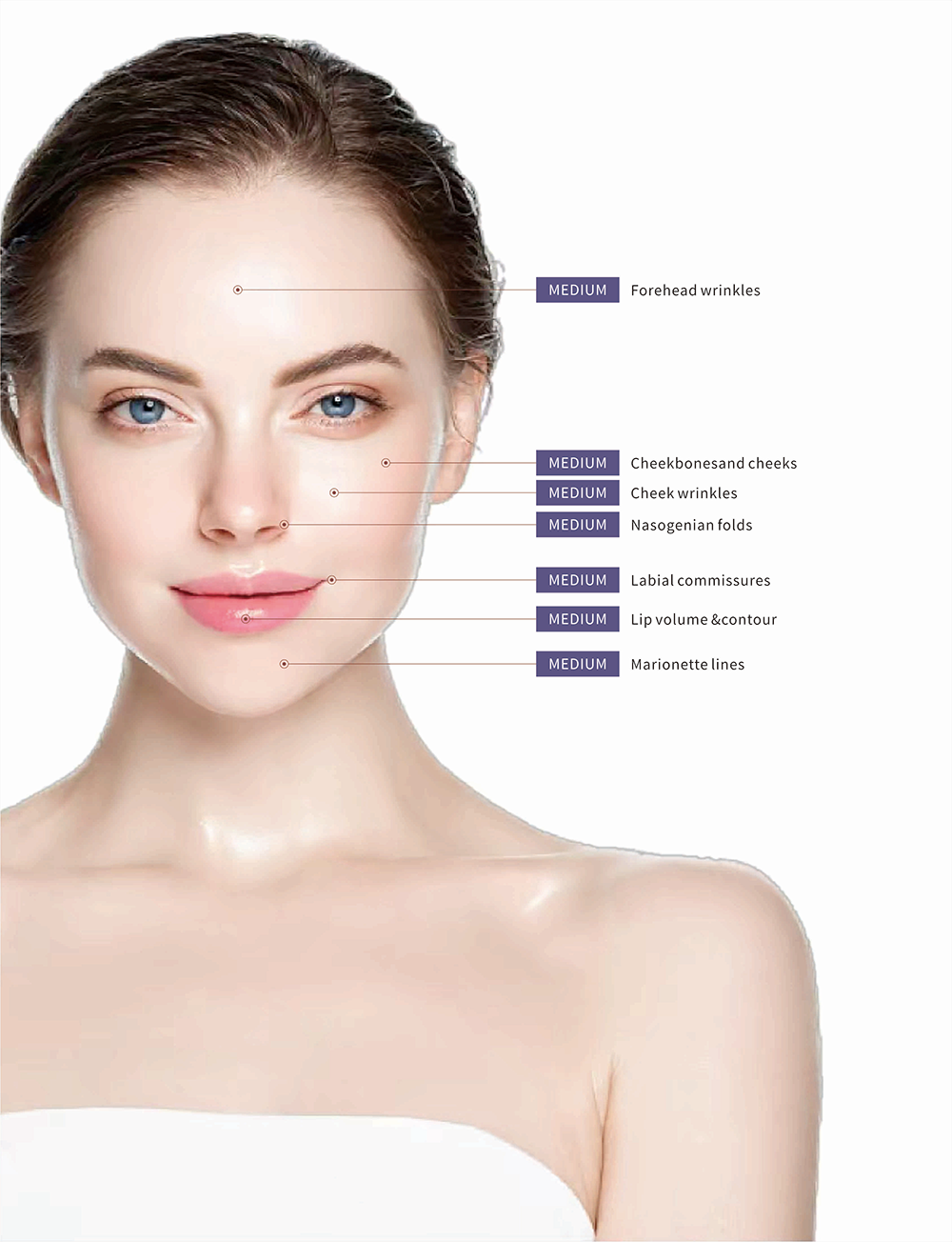
Dermeca MEDIUM Biphasic Hyaluronic Acid Dermal Filler with Lidocaine,Lip Dermal Fillers
Name: Dermeca MEDIUM
Attribute: Crosslinked Hyaluronic Acid Injectable Filler with Lidocaine
Needle Size: 27Gx1pcs +26Gx1pcs
HA Concentration: 24mg / ml
Particle size: 0.15-0.28mm
- Overview
Dermeca MEDIUM Lips Filler achieves lip augmentation by injecting hyaluronic acid, adding volume to the subcutaneous tissue to support sunken lips.
DERMECA crossinked sodium hyaluronate gel for plastic surgenis a sterile, biodegradable, viscoelastic,isotonic, homogenizedand transparent injectable gel implant, it is a kind of stabilizedsodium hyaluronic acid (HA)ofnon-animal origin, intended forsingle use only.This kind ofcross-inked hyaluronic acid provides a prolongedhalf-ife compared with naturally occurring HA, which undertakesthe physiological breakdown in subcutaneous tissue.DERMECA LIGHT for shallow wrinkes around eyes and mouths,MEDlUM is for lip enchancement, middle augmentation, INTENSE is for deeper lines and cheek augmentation.
| Name | Dermeca MEDIUM |
| Attribute | Crosslinked Hyaluronic Acid Injectable Filler with Lidocaine |
| Needle Size | 27Gx1pcs +26Gx1pcs |
| HA Concentration | 24mg / ml |
| lidocaine hydrochloride | 3mg / ml |
| Particle size | 0.15-0.28mm |
| Insertion Depth | surperfacial and middle dermis |
| Effect Duration | 6-10 months |
| Storage Condition | Store at 2-30C.Do not freeze. Protect from light source. |
| Indications for use | It's used for lips defect and augmentation. |
Contraindications
• Patients with a history of hypertrophic or keloid scarring orwith a current streptococcal infection, active skin disease,inflammation or other infections.Patients with a history of autoimmune disease or who arereceiving immune therapy.
• Patients who are known to be hypersensitive to hyaluronic acid.
• This product has not been tested on pregnant orbreastfeeding women, or on children. There is therefore noevidence for it's safety in these groups.
• The existing clinical data has confirmed the safety andeffectiveness of this product for use in adults.There are noclinical data available for using the product in people underthe age of 18. Therefore, the product is not recommendedfor such group.
• Anti-coagulated patients or patients receiving plateletaggregation inhibitors (e.g. ASS) should consult their doctor5.
• The product should be kept out of reach for children.
• Do not use in patients with bleeding disorders.
• Do not use in patients who are taking thrombolytics oranticoagulants, or have taken inhibitors of plateletaggregation in the preceding 2 weeks
• Do not use in patients with hyperplastic scar andstreptococcalinfection.
• Do not use the product together with a laser treatment,chemical peeling, intensive pulse light or dermabrasiontreatment.
• The product should not be used in or near anatomic siteswhere there is active skin disease,inflammation,infection orrelated conditions.
• Do not implant the areas where injection of other productstook place.
Anticipated Adverse Events
Common and short-term, self-limiting reactions after injectiorinclude erythema, swelling, pain, itching and discoloration.
This product equilibrates to the normal pressure of the tissue$.As the pressure of the tissues is sometimes increased (in thecase of oedema) or decreased (in the case of dehydration), asmall change in appearance may occur in these circumstance$.In more serious cases, a short course of oral steroids can beelpful. Patients presenting with this kind of reaction must notbe treated again with this product.
Complication
The estimated incidence of these complication is 0.05%( 1 in2000 patients),which classifies them as to rare events. Noserious adverse events have been reported.